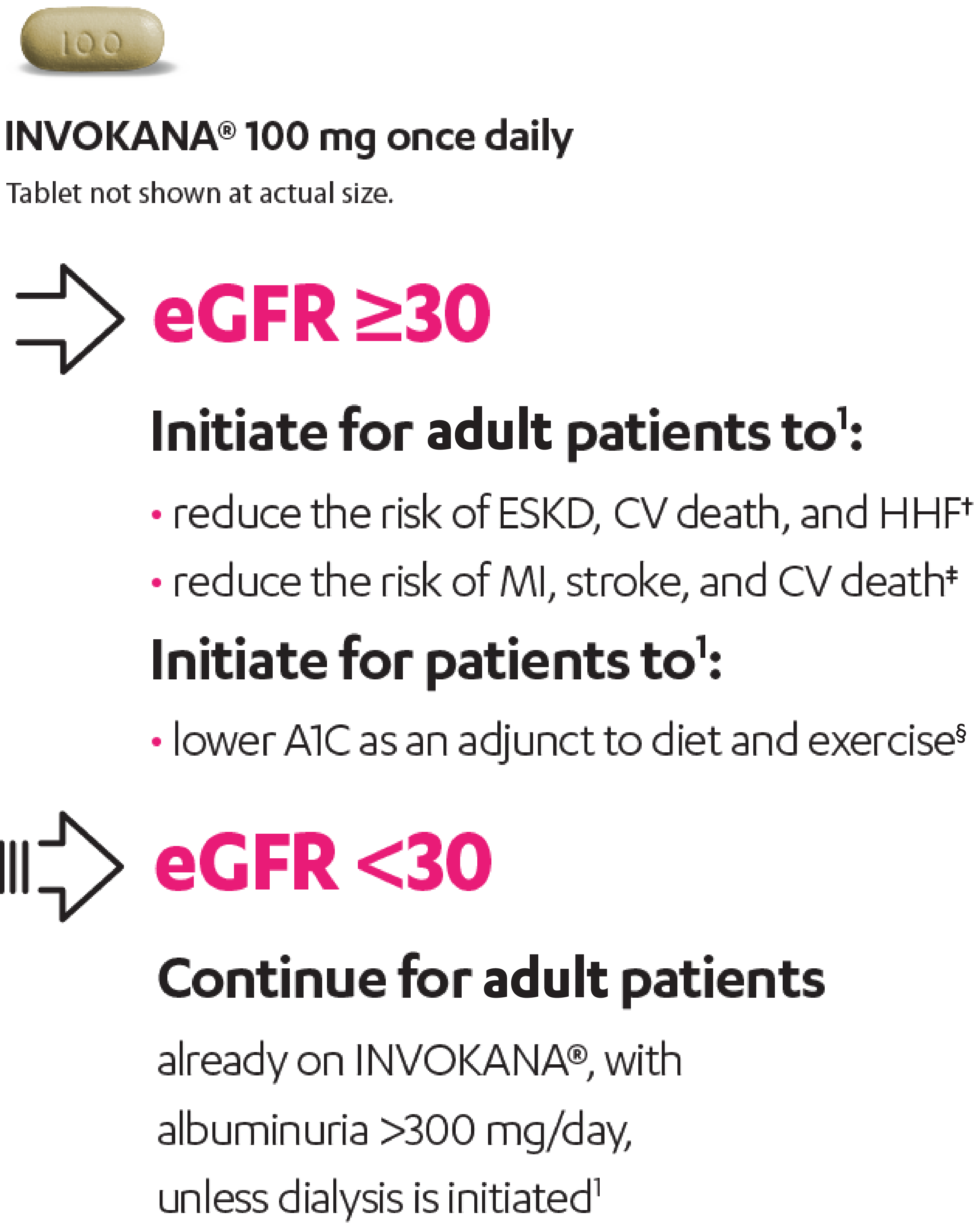
INVOKANA®: the first SGLT2i approved for initiation in adult patients with T2D and an eGFR as low as 30 who have DKD,* established CVD, or are in need of glycemic control1-4
Increase dosage to 300 mg/day in adult patients with an eGFR ≥60 who need additional glycemic control.1
INVOKANA® is not recommended and is likely to be ineffective for glycemic control in adults with T2D with an eGFR <30. INVOKANA® is contraindicated in patients on dialysis.1
eGFR is measured in mL/min/1.73 m2.
*With albuminuria >300 mg/day.
†In adult patients with DKD and albuminuria >300 mg/day.
‡In adult patients with established CVD.
§In adult and pediatric patients aged 10 years and older.
CV=cardiovascular; CVD=cardiovascular disease; DKD=diabetic kidney disease; ESKD=end-stage kidney disease; HHF=hospitalization for heart failure; MI=myocardial infarction; SGLT2i=sodium-glucose co-transporter 2 inhibitor; T2D=type 2 diabetes.
For more information on dosing and administration, please refer to the full Prescribing Information.