Help protect adult patients who have DKD* and T2D
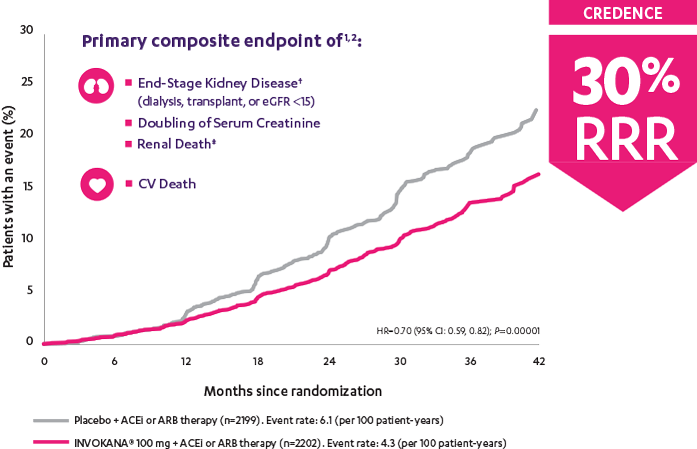
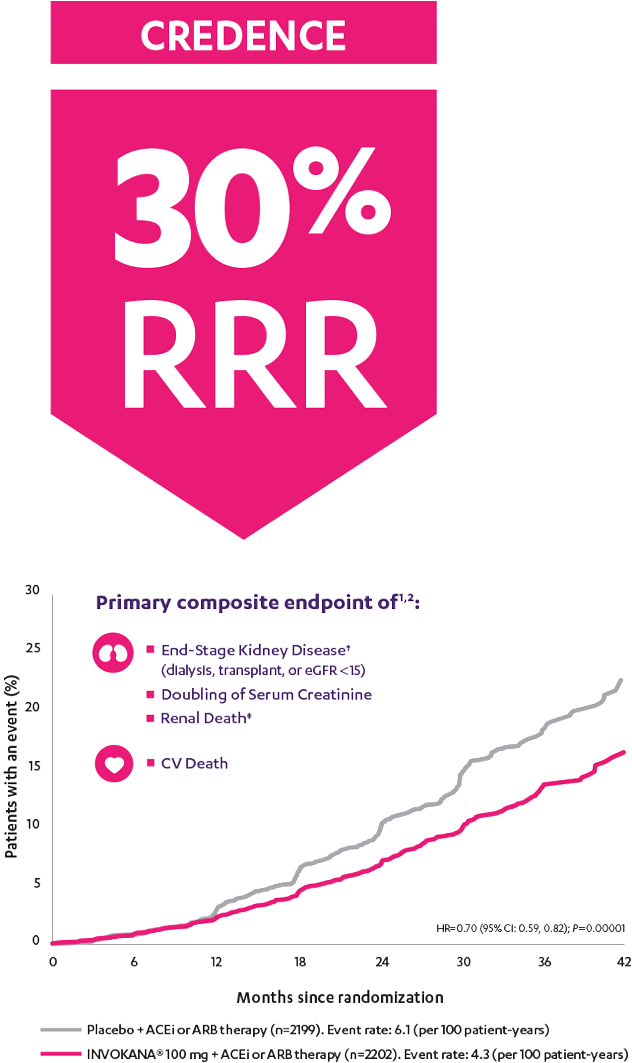
INVOKANA® reduced the relative risk of the primary composite outcome of end-stage kidney disease,† doubling of serum creatinine, and renal‡ or CV death1
The landmark CREDENCE trial ended early after the overall risks and benefits were assessed and clear evidence of efficacy was shown.1
Patients were already taking a maximum tolerated dose of an ACEi or ARB.1
- N=4401
- Mean duration of diabetes: 15.8 years
- Mean baseline eGFR: 56.2 mL/min/1.73 m2
- Median baseline urinary albumin-to-creatinine ratio: 927 mg/g
eGFR is measured in mL/min/1.73 m2.
*With albuminuria >300 mg/day.
†End-stage kidney disease was defined as dialysis for ≥30 days, kidney transplantation, or an eGFR <15 mL/min/1.73 m2 sustained for ≥30 days.
‡There were not enough events to evaluate the risk of renal death (placebo, n=5; INVOKANA®, n=2). INVOKANA® is not indicated to reduce the risk of renal death.
ACEi=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; CREDENCE=Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; CV=cardiovascular; DKD=diabetic kidney disease; HR=hazard ratio; RRR=relative risk reduction; SGLT2i=sodium-glucose co-transporter 2 inhibitor; T2D=type 2 diabetes.
Perkovic et al
CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) was a randomized, double-blind, placebo-controlled, parallel group, multicenter, event-driven clinical trial. The trial compared the effects of INVOKANA® 100 mg vs placebo in 4401 men and women with type 2 diabetes and diabetic kidney disease (described as chronic kidney disease with eGFR 30 to <90 mL/min/1.73 m2 and albuminuria [ratio of albumin to creatinine >300 to 5000 mg/g]) who were already taking a stable, maximum-tolerated, or labeled dose (for ≥4 weeks prior to randomization) of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB). The mean eGFR of patients was 56.2 mL/min/1.73 m2 and the median urinary albumin-to-creatinine ratio was 927 mg/g. The primary efficacy outcome was the composite of end-stage kidney disease (dialysis, transplant, or eGFR <15 mL/min/1.73 m2), doubling of serum creatinine, or renal or cardiovascular (CV) death. Prespecified secondary outcomes included a composite of CV death or hospitalization for heart failure; a composite of heart attack, stroke, or CV death; hospitalization for heart failure; and a composite of end-stage kidney disease, doubling of the serum creatinine level, or renal death.1