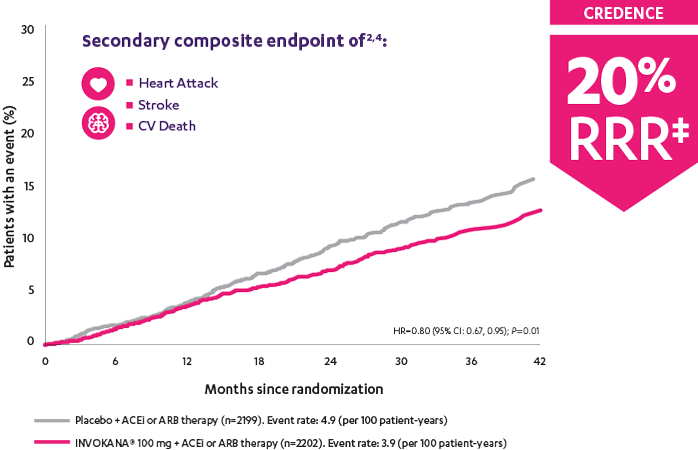
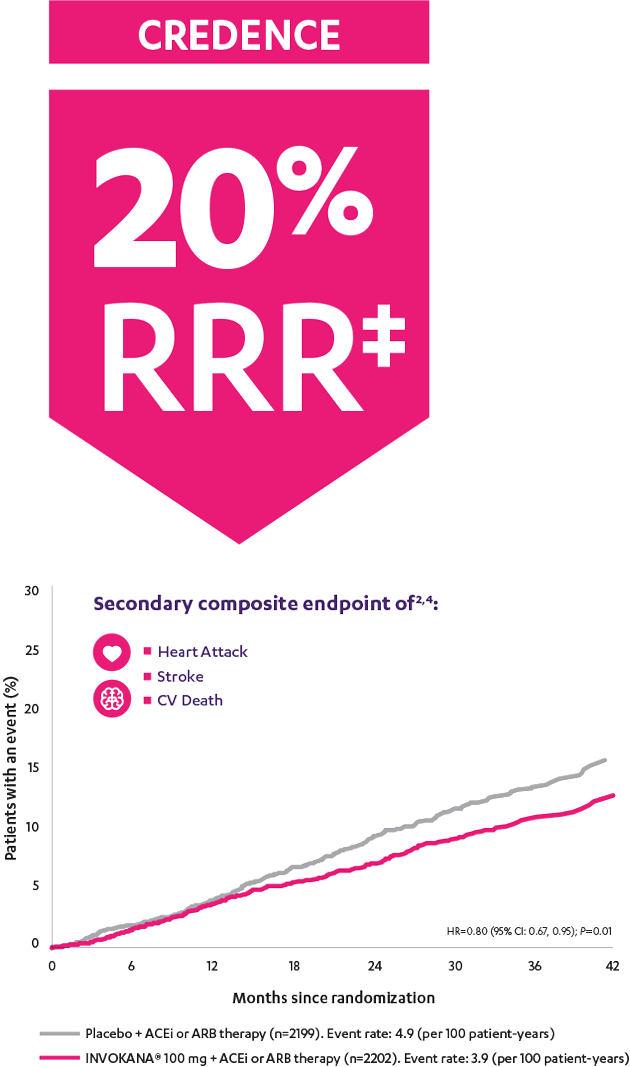
In adult patients who had DKD† and T2D, INVOKANA® 100 mg reduced the relative risk of the composite outcome of heart attack, stroke, or CV death by 20%2
Patients were already taking a maximum tolerated dose of an ACEi or ARB.3
*Prespecified secondary endpoint for CREDENCE; prespecified primary endpoint for CANVAS.
†With albuminuria >300 mg/day.
‡RRR was calculated using the following formula: 100 × (1–HR).
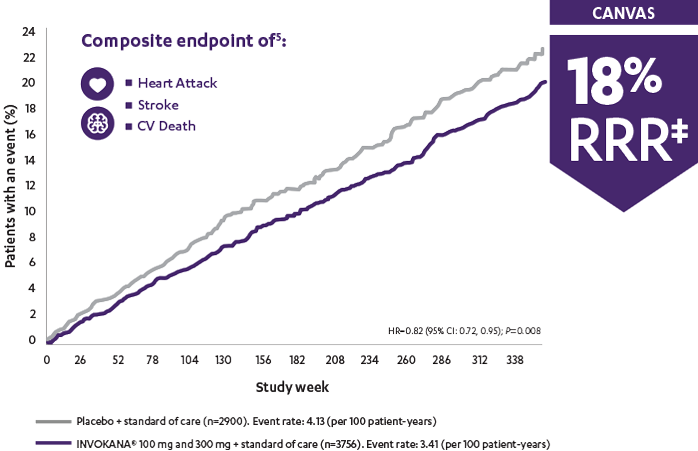
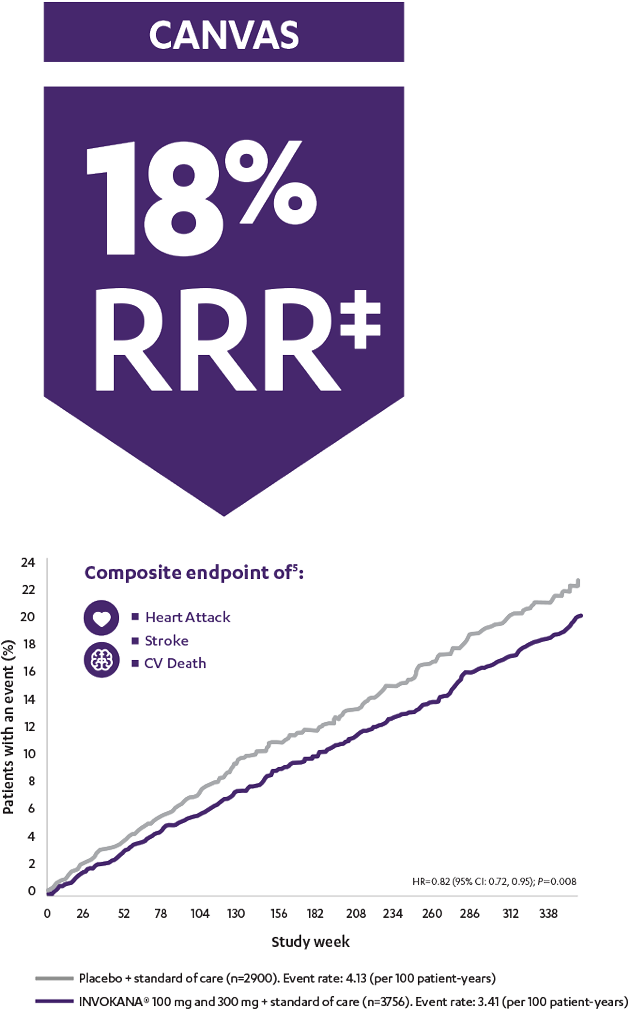
In the longest CV outcomes trial of any SGLT2i, INVOKANA® demonstrated an early and sustained (up to 6.5 years) relative risk reduction‡ of 3-point MACE for adult patients with T2D and CV disease1
‡RRR was calculated using the following formula: 100 × (1–HR).
ACEi=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; CANVAS=Canagliflozin Cardiovascular Assessment Study; CREDENCE=Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; CV=cardiovascular; DKD=diabetic kidney disease; HR=hazard ratio; MACE=major adverse cardiovascular events; RRR=relative risk reduction; SGLT2i=sodium-glucose co-transporter 2 inhibitor; T2D=type 2 diabetes.
Perkovic et al
CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) was a randomized, double-blind, placebo-controlled, parallel group, multicenter, event-driven clinical trial. The trial compared the effects of INVOKANA® 100 mg vs placebo in 4401 men and women with type 2 diabetes and diabetic kidney disease (described as chronic kidney disease with eGFR 30 to <90 mL/min/1.73 m2 and albuminuria [ratio of albumin to creatinine >300 to 5000 mg/g]) who were already taking a stable, maximum-tolerated, or labeled dose (for ≥4 weeks prior to randomization) of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB). The mean eGFR of patients was 56.2 mL/min/1.73 m2 and the median urinary albumin-to-creatinine ratio was 927 mg/g. The primary efficacy outcome was the composite of end-stage kidney disease (dialysis, transplant, or eGFR <15 mL/min/1.73 m2), doubling of serum creatinine, or renal or cardiovascular (CV) death. Prespecified secondary outcomes included a composite of CV death or hospitalization for heart failure; a composite of heart attack, stroke, or CV death; hospitalization for heart failure; and a composite of end-stage kidney disease, doubling of the serum creatinine level, or renal death.3
Neal et al
The CANVAS Program was an integrated analysis of 2 trials (the CANVAS trial and the CANVAS-R trial) with a total of 10,142 men and women with type 2 diabetes. Of the participants, 96.0% completed the trial and vital status was confirmed for 99.6%. The mean follow-up for the CANVAS Program was 188.2 weeks, while the length of follow-up was 295.9 weeks and 108.0 weeks in the CANVAS and CANVAS-R trials, respectively. Participants were either ≥30 years of age with a history of symptomatic atherosclerotic cardiovascular disease or ≥50 years of age with ≥2 risk factors* for cardiovascular disease. The primary efficacy outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke.1
*≥2 of the following risk factors for CVD: duration of diabetes ≥10 years, systolic blood pressure >140 mm Hg while they were receiving ≥1 antihypertensive agents, currently smoking, microalbuminuria or macroalbuminuria, or HDL cholesterol level <1 mmol/L (38.7 mg/dL).
Mahaffey et al
The CANVAS Program was a double-blind comparison of the effects of INVOKANA® vs placebo from an integrated analysis of 2 large-scale trials (the CANVAS trial and the CANVAS-R trial) including 10,142 men and women with type 2 diabetes who were either ≥30 years of age with a history of symptomatic atherosclerotic cardiovascular events or ≥50 years of age with ≥2 risk factors for cardiovascular disease. The established CVD subgroup included ~70% of all patients. The primary efficacy outcome for these analyses was the composite of cardiovascular mortality, nonfatal Ml, or nonfatal stroke.5