Dosing options and tailored therapy to fit individual patient needs1
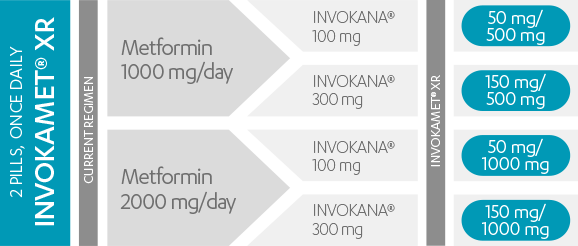
INVOKAMET® XR is available in 4 strengths1
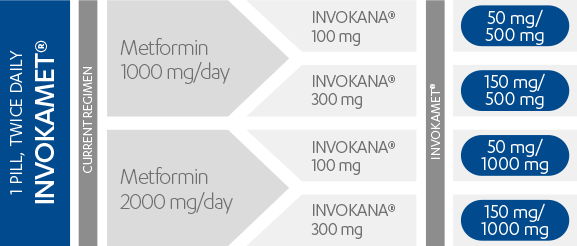
INVOKAMET® is available in 4 strengths1
For more information on dosing and administration for INVOKAMET® XR or INVOKAMET®, please see the full Prescribing information and Medication Guide.

